In our other article, we introduced activated carbon; its structure, what it is made from, the main forms and how it is produced. In this article, we explain how activated carbon works.
Carbon Adsorption?
Note that there is an important and distinct difference between ADsorption and ABsorption:

Adsorption is caused by London Dispersion Forces, which is a type of Van der Waals or electrostatic force that exists between all molecules. Van der Waals forces are non-specific and are relatively weak electric forces that attract neutral molecules to one another in liquids, air and gases.
London Dispersion Forces are short, ranged attraction forces and therefore quite sensitive to the distance between the surface of the activated carbon and the molecule being adsorbed. However, these forces are additive.
This means the adsorption force at the adsorption site is the sum of all the interactions between the carbon atoms and the molecule to be adsorbed. The short range and additive nature of these forces means that activated carbon is considered to have the strongest physical adsorption forces and the highest adsorption volume of any known material.
Therefore, The strongest adsorption forces occur when the distance between the carbon platelets and the molecule to be adsorbed is very close, so there is the strongest adsorption energy.
Adsorption is the deposition of compounds (adsorbates) from water or solution or the condensation of gases onto the surface of the activated carbon (or adsorbent). These adsorbates undergo a phase change on adsorption because they are so much lower in energy once adsorbed. Adsorption therefore really takes place through energetic interactions and not through a process of molecular sieving.
However, activated carbon does not swell like an ion exchange resin or sponge. This is because all of the work happens deep inside the carbon structure where the adsorbing molecule displaces any water molecules held on the internal surface area.
Contaminant molecules are effectively held in place on the interior carbon surface and removed because of the numerous interactions made in the carbon “pore”.
Activated Carbon Structure
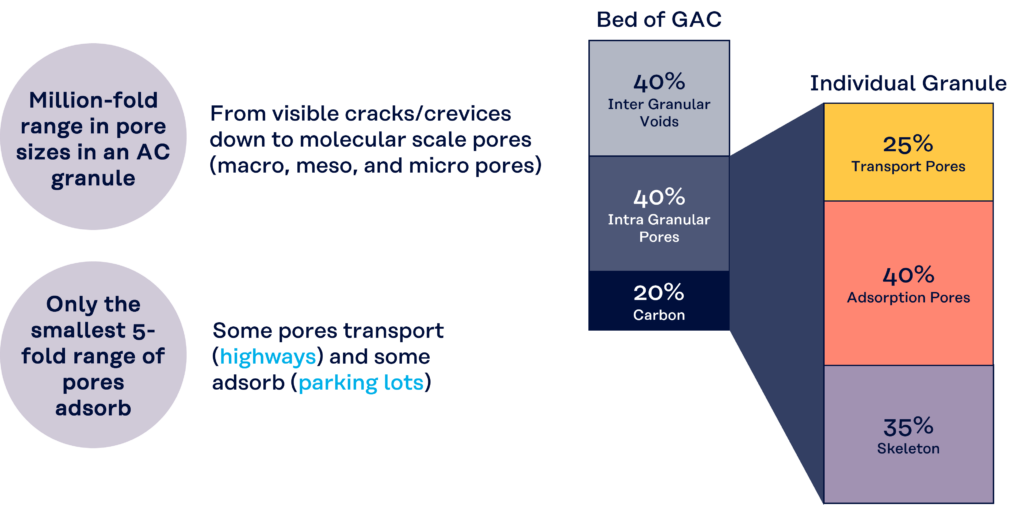
A bed of activated carbon consists of approximately
- 40% which are the voids between the carbon particles that are the drainable volume if the carbon bed is in solution.
- 20% is considered the volume of the carbon particle itself that comprises the carbon skeleton.
- 40 % is considered the overall ‘pore volume’ within the carbon particle which is the non-drainable volume.
Then taking just the individual carbon granule or particle itself, this then consists of approximately: –
- 35% as then the carbon skeleton volume,
- 25 % as the ‘transport pore’ volume
- 40 % as the ‘adsorption pore’ volume
The ‘transport pore’ volume comprises large pores which vary in size from the largest being 0.1 microns to visible cracks and crevices in the particle of 0.1 mm.
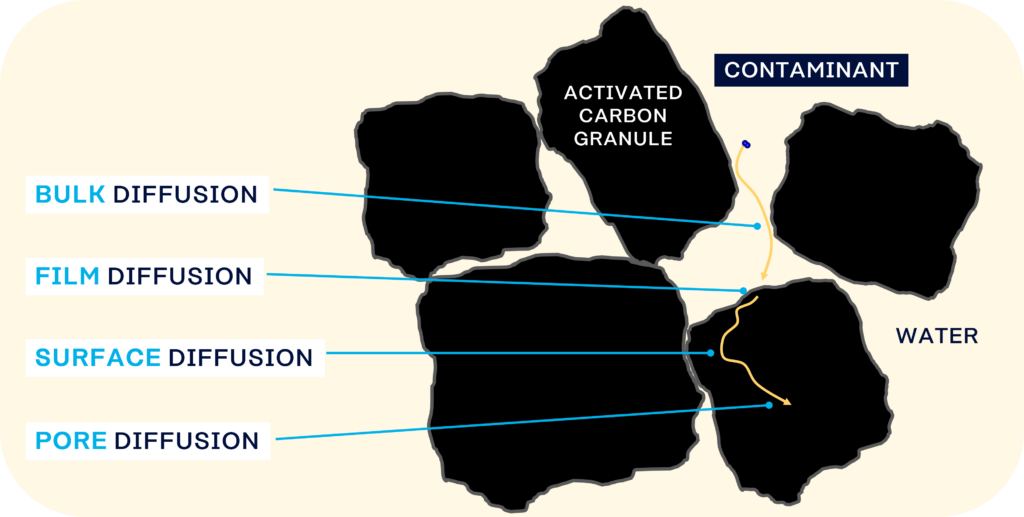
These ‘transport pores’ act as a diffusion pathway to transport the contaminant from outside the carbon structure, into the particle and to the adsorption site. They contain concentration or pressure gradients which drive the pore diffusion process and so the contaminant into the adsorption sites.
These pores are much larger than the largest adsorption pores and are not able to adsorb contaminants, even at near-saturation conditions. Therefore, any solution in these pores is considered potentially recoverable.
The illustration below shows an adsorbing compound (adsorbate) in a liquid which diffuses through the activated carbon bed, through the particle itself and then into the adsorption site.
The ‘adsorption pores’ are the finest pores in the carbon structure and those parts of the carbon structure that have the adsorption capability. These adsorption pores are the gaps, cracks and crevices between the graphite plates where there are sufficient intermolecular forces for adsorption to occur. The widths of this structure are of molecular dimensions and no greater than 10 to 50nm.
Adsorption only occurs in the adsorption pore regions and at saturation, all adsorption pore regions are then filled. Hence any solution in these pores is considered non-recoverable.
The molecule to be adsorbed needs to be close to a carbon plate in order to adsorb. Once the adsorbate is more than a few molecular diameters away from the carbon surface, adsorption no longer occurs.
How Does Activated Carbon Work?
Activated Carbon works by a process of adsorption due to its pore structure, high surface area, and high surface reactivity.
The key factors that affect adsorption onto activated carbon are:
- Concentration – the weight % loading on the carbon increases as the concentration of the inlet contaminant increases.
- Structural Complexity – the more complex structures adsorb more readily
- Molecular Weight – the higher the molecular weight of the contaminant the better it is adsorbed
- Temperature – the higher the influent temperature, the more poorly the contaminant is adsorbed.
In the liquid phase, the molecules of the compound to be removed go from the bulk liquid to being adsorbed into the pores of the activated carbon, in a semi-liquid state. The additional factor that affects adsorption in the liquid phase is
- Solubility – the higher the solubility, the more difficult it is to adsorb
Therefore, the adsorbability of an organic molecule increases with increasing molecular weight and decreasing solubility.
The vapour or gas phase is a condensation process where the adsorption forces condense the molecules of the compound into a liquid. This can then be removed from the air or gas phase within the pores of the activated carbon. The additional factors that affect adsorption in the vapour phase are:
- Vapour Pressure – lower vapour pressure compounds are more adsorbable than high vapour pressure compounds.
- Relative Humidity – a lower relative humidity leads to better adsorption
- Polarisability – the higher the polarity, the more difficult it is to adsorb
However, the actual life of a carbon bed is dependent on many other factors. These include the type of carbon selected, the specific contaminant and concentration for removal, and then the required treatment level for the contaminant or contaminants.
In addition, the overall application conditions are important. These include what other compounds may be present and their concentrations and the system design installed or planned which includes the EBCT – empty bed contact time.
What Compounds Can be Adsorbed onto Activated Carbon?
All compounds are adsorbable to some extent on activated carbon. Those compounds that are readily adsorbed are aromatic and chlorinated compounds, PFAS, VOCs, and colour bodies.
In practice, activated carbon is used primarily for the adsorption of organic compounds and some inorganic compounds when an impregnated activated carbon is used.
Depending on the application, different types of activated carbon may be required. It’s important to select the appropriate grade and size concerning the pollutant intended to be removed or the degree of purity you wish to achieve.
At Chemviron, we have over 80 years of experience designing, manufacturing, and implementing activated carbon solutions. during which, we have developed a library of field data and performance information.
Using this data and our proprietary models, Chemviron is better able to predict the likely activated carbon performance. We, therefore, can customise more effective treatment solutions based on the energy of adsorption concept, rather than the now older micro-, meso- and macropore definitions.
See the following sections where the specific compounds to be removed are covered in more detail.








